valence electron of molybdenum|Molybdenum Valence Electrons : Cebu The total number of electrons in molybdenum is forty-two. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons . Tingnan ang higit pa Lottery Sambad is a big game that many people in India love to play. It is also known as dear lottery. It is a lucky draw where you can win lots of money and prizes. Every day, three different draws take place, and people eagerly wait for the results. It is a thrilling moment when the numbers are announced on television and radio.
PH0 · molybdenum
PH1 · Valence Electrons Chart for All Elements
PH2 · Technical data for the element Molybdenum in the Periodic Table
PH3 · Periodic Table of Elements: Molybdenum
PH4 · Molybdenum Valence Electrons
PH5 · Molybdenum Electron Configuration (Mo) with Orbital Diagram
PH6 · Molybdenum Electron Configuration (Mo) with Orbital
PH7 · Molybdenum (Mo)
PH8 · Molybdenum
PH9 · Complete Electron Configuration of Molybdenum (Mo, Mo3+)
River Belle online casino offers a first deposit bonus of 100% of the deposit amount, up to a maximum of $800. The bonus is distributed as follows: . Playing games on Riverbelle casino mobile is easy and fun. River Belle has made its games available on Android so that you can play anytime. Before you play the game, get acquainted with it .Bagmane Tech Park - Laurel (Block C) is a Tech Park in C V Raman Nagar, Bangalore with a total built-up area of ~2,40,000 sq. ft. . The building covers a total built-up area of ~2,09,135 sq. ft. and a floor plate .
valence electron of molybdenum*******The ground state electron configuration of molybdenum is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d5 5s1. This electron configuration shows that the last shell of molybdenum has an electron and the d-orbital has a total of five electrons. Therefore, the valence electronsof molybdenum are six. The . Tingnan ang higit paThe total number of electrons in molybdenum is forty-two. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof . Tingnan ang higit pa
Mar 23, 2023 The diagram draws and represents the actual numbers of valence electrons. Having the proper understanding of Molybdenum valence electrons will .
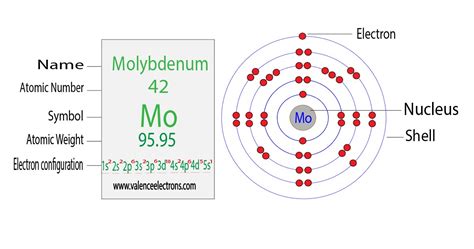
Molybdenum disulfide is used as a lubricant additive. Other uses for molybdenum include catalysts for the petroleum industry, inks for circuit boards, pigments and electrodes. .In its pure form, molybdenum is a silvery-grey metal with a Mohs hardness of 5.5 and a standard atomic weight of 95.95 g/mol. It has a melting point of 2,623 °C (4,753 °F), sixth highest of the naturally occurring elements; only tantalum, osmium, rhenium, tungsten, and carbon have higher melting points. It has one of the lowest coefficients of thermal expansion among commercially used metals.

Molybdenum is a chemical element of the periodic table with chemical symbol Mo and atomic number 42 with an atomic weight of 95.951 u and is classed as transition metal .
Valence Electrons: 4d 5 5s 1 Electron Dot Model. Chemical Properties of Molybdenum. Electrochemical Equivalent: 0.8949g/amp-hr; Electron Work Function: 4.6eV; .
Technical data for Molybdenum. Click any property name to see plots of that property for all the elements. Notes on the properties of Molybdenum: Specific Heat: Value given for .
Molybdenum Number of Valence Electrons. As we have explained above that the Molybdenum is having the Valence of 0,2+,3+,4+,5+ and 6+. The simple explanation behind this Valence of .IUPAC Standard InChIKey:ZOKXTWBITQBERF-UHFFFAOYSA-N Copy. CAS Registry Number: 7439-98-7. Chemical structure: This structure is also available as a 2d Mol file. . Molybdenum's valence electrons are found in the outermost shell, more precisely in the 5s subshell. Molybdenum dichloride (\( MoCl_2\)), for instance, has a valency of 2, whereas molybdenum trioxide (\( MoO_3 \)) has a valency of 6. In certain compounds, molybdenum can also have a valency of 3, 4, or 5. . Molybdenum Valence Electrons Dot Diagram. You can consider studying the Mo valence electrons with its dot diagram. The diagram draws and represents the actual numbers of valence electrons. Having the proper understanding of Molybdenum valence electrons will help in understanding the chemical bonding. So, the Lewis dot .valence electron of molybdenum Molybdenum Valence Electrons The properties of an element can be determined by electron configuration. Also, the valency, valence electrons, and ionic properties of the elements are determined by the . The last electron of . This chemical element is capable of holding the one Electron in the 5S and the further the five Electrons in the 4d shells. This whole structure gives Molybdenum the valence of 0,2+,3+,4+,5+ and 6+. Molybdenum Number of Valence Electrons. As we have explained above that the Molybdenum is having the Valence of 0,2+,3+,4+,5+ . You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.Molybdenum is the 42nd element in the periodic table and has a symbol of Mo and atomic number of 42. It has an atomic weight of 95.95 and a mass number of 98. Molybdenum has forty-two protons and fifty-six neutrons in its nucleus, and forty-two electrons in five shells. It is located in group six, period five and block d of the periodic table. Molybdenum has six valence electrons. The atomic number of molybdenum is 42, and its electron configuration is 1s22s22p63s23p63d104s24p64d55s1 or 2, 8, 18, 13, 1 .
Molybdenum is a chemical element of the periodic table with chemical symbol Mo and atomic number 42 with an atomic weight of 95.951 u and is classed as a transition metal. . Valency electrons : 2,3,4,5,6: Bohr model: Electron shell for Molybdenum, created by Injosoft AB Mo. Figure: Shell diagram of Molybdenum (Mo) atom. Orbital Diagram. 1s . The second electron will also enter the p y orbital in the clockwise direction and the third electron will also enter the p z orbital in the clockwise direction.. Now, when the fourth electron wants to enter the p-subshell, then it will enter the p x orbital in the anti-clockwise direction.. The fifth electron will also enter the p y orbital in the anti-clockwise . (6)====Molybdenum is within group 16. If in group 1 then it has 1, if group 2 then it has 2. If in the teens, drop the 1. So this element is in group 16 so it has 6 valence electrons. Try Chlorine . Electron binding energies for molybdenum. All values of electron binding energies are given in eV. The binding energies are quoted relative to the vacuum level for rare gases and H 2 , N 2 , O 2 , F 2 , and Cl 2 molecules; relative to the Fermi level for metals; and relative to the top of the valence band for semiconductors. Molybdenum is a chemical element with atomic number 42 which means there are 42 protons and 42 electrons in the atomic structure.The chemical symbol for Molybdenum is Mo. Electron Configuration and Oxidation States of Molybdenum. Electron configuration of Molybdenum is [Kr] 4d5 5s1. Possible oxidation states are .valence electron of molybdenumProtons and Neutrons in Molybdenum. Molybdenum is a chemical element with atomic number 42 which means there are 42 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to .
Molybdenum is the element with atomic number 42 and is having electronic configuration [K r] 5 s 1 4 d 5, where atomic no. of Kr = 36. Thus no. of valence electrons is 1, which is no of an electron in 5s subshell.
The electron configuration of molybdenum can be represented as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d5. This configuration indicates that molybdenum has five valence electrons, which are located in the 4d orbital. The presence of these valence electrons in the 4d orbital is what gives molybdenum its unique properties.
Login as Buyer Login as CSO Login as Supplier Login to Virtual Store. Other User. HELP. FAQs; PhilGEPS 1.5; PhilGEPS 2.0; PBB Compliance; PhilGEPS 1.5. PhilGEPS 1.5 is an enhancement of the existing system that aims to improve reliability, performance and increase number of users accessing the site.
valence electron of molybdenum|Molybdenum Valence Electrons